Jan 5 2017
Scientists have developed a new method for producing microscale machines using biomaterials that are safe to be implanted in the human body. The team has been headed by Sam Sia, Biomedical Engineering Professor at Columbia Engineering. Sia has developed a new method for using hydrogels, which are biocompatible materials that have been under study for many decades. The method involves stacking the soft material in layers to produce devices that contain freely moving, three-dimensional parts. The research work—exhibiting a fast manufacturing technique which Sia terms “implantable microelectromechanical systems” (iMEMS)—was reported online in the journal Science Robotics, on 4 January 2017.
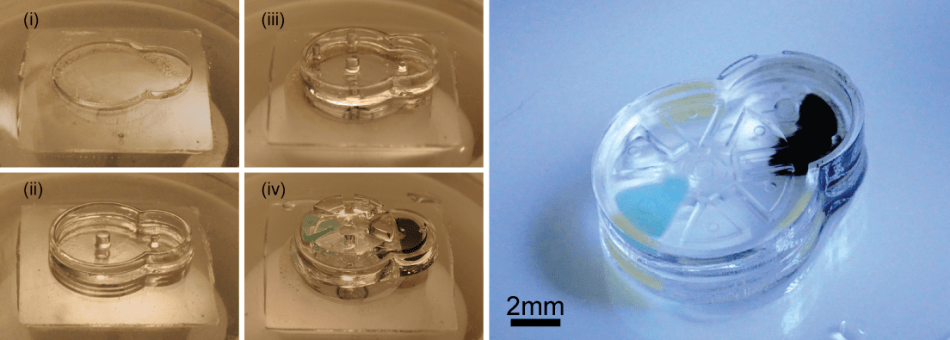 Fabrication and complete assembly of a Geneva drive device using the iMEMS method. The left panel shows the layer-by-layer fabrication of support structures and assembly of gear components. The image on the right shows the complete device after the layers have been sealed. Credit: Photo by Sau Yin Chin/Columbia Engineering.
Fabrication and complete assembly of a Geneva drive device using the iMEMS method. The left panel shows the layer-by-layer fabrication of support structures and assembly of gear components. The image on the right shows the complete device after the layers have been sealed. Credit: Photo by Sau Yin Chin/Columbia Engineering.
The researchers made good use of the distinctive mechanical characteristics of hydrogels to develop a “locking mechanism” to enable accurate actuation and movement of freely moving parts, thus allowing the parts to function as valves, pumps, manifolds, drug delivery systems, and rotors. The team succeeded in adjusting the biomaterials within a broad range of diffusive and mechanical properties and in controlling the biomaterial after being implanted without the need for a sustained power supply (i.e. toxic battery). The team then tested the payload delivery in a bone cancer model and discovered that when doxorubicin release was from the device triggered for more than 10 days, it led to low toxicity and high treatment efficacy, of the order of 1/10th of the standard systemic chemotherapy dose.
Sam Sia: Biomechanical Machines
Magnetic actuation of the Geneva drive device. A magnet is placed about 1cm below and without contact with the device. The rotating magnet results in the rotational movement of the smaller driving gear. With each full rotation of this driving gear, the larger driven gear is engaged and rotates by 60º, exposing the next reservoir to the aperture on the top layer of the device. — Video courtesy of Sau Yin Chin/Columbia Engineering
Overall, our iMEMS platform enables development of biocompatible implantable microdevices with a wide range of intricate moving components that can be wirelessly controlled on demand and solves issues of device powering and biocompatibility. We’re really excited about this because we’ve been able to connect the world of biomaterials with that of complex, elaborate medical devices. Our platform has a large number of potential applications, including the drug delivery system demonstrated in our paper which is linked to providing tailored drug doses for precision medicine.
Sia, who is also a member of the Data Science Institute.
Instead of containing moving parts, many of the prevalent implantable microdevices include static components, and they have limited biocompatibility because of the need for batteries or similar toxic electronics. To overcome this challenge, the research team led by Sia worked for more than eight years.
Hydrogels are difficult to work with, as they are soft and not compatible with traditional machining techniques. We have tuned the mechanical properties and carefully matched the stiffness of structures that come in contact with each other within the device. Gears that interlock have to be stiff in order to allow for force transmission and to withstand repeated actuation. Conversely, structures that form locking mechanisms have to be soft and flexible to allow for the gears to slip by them during actuation, while at the same time they have to be stiff enough to hold the gears in place when the device is not actuated. We also studied the diffusive properties of the hydrogels to ensure that the loaded drugs do not easily diffuse through the hydrogel layers.
Sau Yin Chin, who is the lead author of the study, and also a colleague of Sia.
The research team polymerized sheets of gel using light. They also included a stepper mechanization for controlling the z-axis and patterning the sheets one layer at a time, thus ensuring three-dimensionality. Through z-axis control the researchers could produce composite structures within a single hydrogel layer and simultaneously manage the thickness of each layer throughout the entire fabrication process. They could accurately align the stack multiple layers. As they were able to polymerize the sheets layer by layer, one after the other, the complex structure was created within a timeframe of 30 minutes.
The iMEMS method discovered by Sia addresses the following fundamental considerations while manufacturing biocompatible micromachines, microdevices, and microrobots: the way to manufacture small biocompatible moveable components without using silicon, which has limited biocompatibility; how to power small robotic devices without employing toxic batteries; and how to carry out wireless communication after implantation (radio frequency microelectronics are relatively large, need power, and are not biocompatible). The researchers were successful in triggering the iMEMS device to emit additional payloads over a time period of few days to few weeks once implanted. They could also achieve accurate actuation by making use of magnetic forces to induce gear movements which, consequently, bent structural beams formed from hydrogels with highly tunable characteristics. (It is to be noted that Magnetic iron particles are approved by the US Food and Drug Administration (FDA) and are commonly used for human use as contrast agents.)
The team worked with Francis Lee—an orthopedic surgeon at Columbia University Medical Center at the time of the research—to test the drug delivery system on mice suffering from bone cancer. The iMEMS system was successful in delivering chemotherapy adjacent to the cancer. It also limited tumor growth and exhibited lower toxicity when compared to chemotherapy administered to the entire body.
These microscale components can be used for microelectromechanical systems, for larger devices ranging from drug delivery to catheters to cardiac pacemakers, and soft robotics. People are already making replacement tissues and now we can make small implantable devices, sensors, or robots that we can talk to wirelessly. Our iMEMS system could bring the field a step closer to developing soft miniaturized robots that can safely interact with humans and other living systems.
Sia
The research titled “Additive manufacturing of hydrogel-based materials for next-generation implantable medical devices,” was supported by an NIH R01 grant (HL095477-05), NSF CAREER award, and NSF ECCS-1509748. Chin was supported by the National Science Scholarship (PhD) awarded by the Agency for Science, Technology and Research (Singapore). The research team has a pending patent.