Nov 28 2012
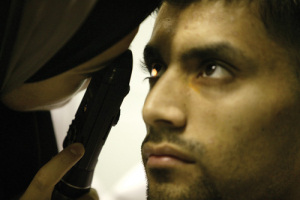
The radiotherapy is delivered by a non-invasive robotic device called IRay® Radiotherapy System which uses low-level radiation, similar to a dental x-ray. Patients treated with the IRay device sit at the machine with their chin on a chin rest.
 Wet AMD affects 250,000 people in the UK alone
Wet AMD affects 250,000 people in the UK alone
A contact lens is placed on the surface of their eye, and a robot tracks any eye movement via the lens and maintains stability. This allows a controlled dose of radiation to be precisely delivered into the eye on the macula.
The team, led by Mr Tariq Aslam, Consultant Ophthalmologist at Manchester Royal Eye Hospital and Honorary Senior Lecturer at The University of Manchester, conducted an initial clinical trial of IRay in 2011. This trial, called INTREPID, combined radiation therapy with the standard AMD treatment of injections into the eye. The number of injections needed within the treatment group was reduced by 32%, compared to people receiving the standard treatment of a monthly injection into the eye. Certain patients achieved an even higher reduction in injections of up to 50%.
The Manchester team then worked with the developer of IRay, US-based company Oraya Therapeutics, to make the device even easier to use and to further reduce treatment times for patients and clinical staff. They are now running a clinical trial over the next few months with 10 patients using the enhanced IRay workflow alongside injections.
Patients only need one session of IRay treatment, which potentially can reduce or even remove the need for the regular injections.
AMD affects thousands of people and is the leading cause of blindness in the UK for people over 65 years of age. Wet AMD is an aggressive form of the disease, affecting 250,000 people in this country, and if left untreated can quickly lead to loss of central vision.
“Treatments such as this under clinical trial conditions represent a significant logistical challenge and Iain McLean, our research manager, and Ekaterina Varimezova-Georgieva, our research co-ordinator, were instrumental to achieving the treatments,” said Mr Aslam.
“This novel therapy has to be co-ordinated with the timing of the patients’ standard treatment and the availability of Oraya’s specialist engineers, who come over from the US to provide technical support. Both Manchester patients were delighted to have been given this novel therapy, which showed significant improvements to standard AMD care in earlier randomised studies.”
Mrs Stella Chandler from North Manchester is one of the first two patients treated with IRay in this new trial. She said: “I joined the trial as I was driven by optimism about the potential benefits of the treatment in my own case, and also because this research could help other people. Having regular injections into one or both eyes is a traumatic process, so anything that reduces the frequency of the injections will be a positive result.”
Local funding support helped the trial to take place in Manchester. Debbie Vinsun, Greater Manchester Comprehensive Local Research Network (GM CLRN) senior manager, said: "Congratulations to Tariq and the ophthalmology team at the Royal Eye Hospital. They have used National Institute for Health Research GM CLRN funding to support staff with different skills, such as a photographer, nurses and a co-ordinator, to ensure that patients in Greater Manchester are getting access to treatments which are not only cutting edge but show promising improvements to the current treatments."
Mr Aslam added: "IRay is an exciting new technology that targets one of the most common causes of blindness in the UK. If the initial results are borne out in this further trial, then a majority of patients will have something to look forward to - an easily administered, one-off treatment that maintains or improves vision, and fewer injections into their eye."
Source: http://www.manchester.ac.uk/