Jun 10 2019
MUSA is the first surgical robot in the world to be used in open microsurgery. This robotic system is commercially and clinically available following the recent issuance of medical device CE-mark by DEKRA, a notified body.
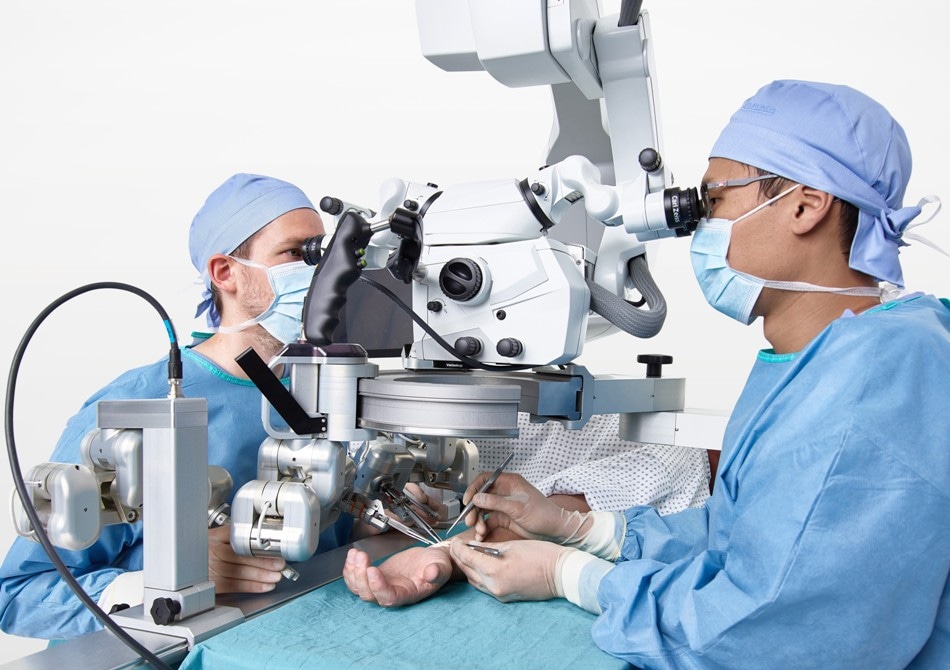 Surgeons using Microsure's MUSA during a microsurgical procedure in a patient's arm. Photo courtesy of Microsure.
Surgeons using Microsure's MUSA during a microsurgical procedure in a patient's arm. Photo courtesy of Microsure.
MUSA from Microsure has been designed by a group of engineers and microsurgeons. It is a high-precision robotic assistant that can be used with existing workflow, operating techniques, instruments, and other operating room equipment. It improves surgical performance by stabilizing and lowering the movements of a surgeon during complicated microsurgical procedures on a sub-millimeter scale.
For instance, Microsure’s MUSA facilitates lymphatic surgery on <0.3-mm-diameter lymph vessels. Plastic surgeons from Maastricht University Medical Center+ used MUSA for the first time for the surgical treatment of lymphedema in a patient. This world’s foremost super-microsurgical intervention with “robot hands” was carried out in 2017. Currently, Maastricht University Medical Center+ is performing many follow-up clinical research projects using MUSA in various fields of microsurgery. It is anticipated that other European hospitals will be starting clinical research this year.
Microsurgery Procedures with MUSA
Complex surgeries on small tissue structures such as lymphovenous anastomosis (LVA) surgery, free flap surgery, pediatric vascular surgery, and finger and hand replantation highly considerably benefit from Microsure’s MUSA when human accuracy is the limiting factor due to poor accessibility, physiological tremor, or fatigue. Many studies and pilots have confirmed a safe and effective clinical use of the device.
ISO 13485 Certification
Microsure also obtained the ISO 13485 certificate, which proved to be another major milestone. The ISO certification guarantees that Microsure fulfills the highest standards in quality management and regulatory compliance procedures to manufacture, develop, and test its products and services. ISO 13485:2016 is the most recent update to ISO requirements for a complete quality management system for designing and manufacturing medical devices.